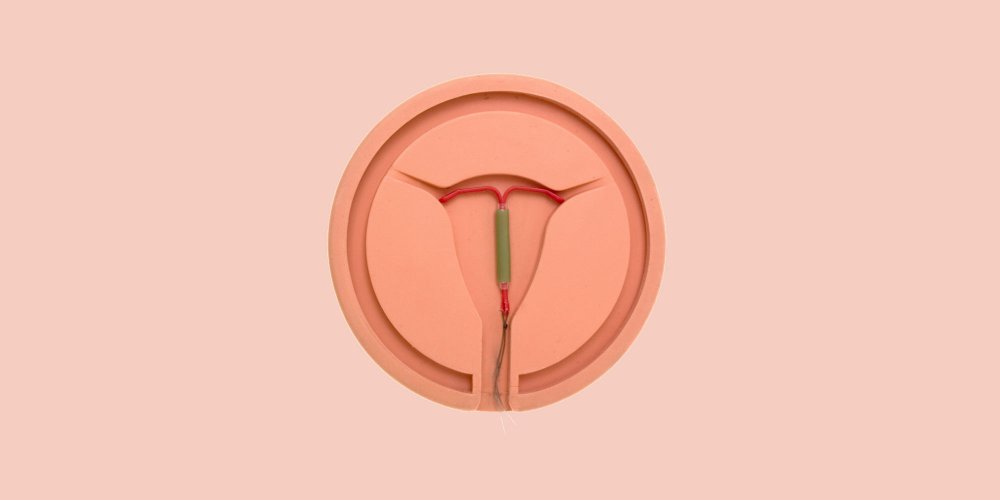
More than 2700 statements. This is the number recorded by the ANSM between May 15 and August 4, 2017 concerning various adverse effects attributed to the Mirena IUD , a levonorgestrel intrauterine device.
Carabine migraines, hair loss, ovarian cysts, but also anxiety and even depression have been reported by hundreds of women using this method of contraception.
At the root of this tidal wave of reports, the Facebook group "Victims of the hormonal IUD Mirena", which has notably encouraged women who have encountered problems with this IUD to report to the ANSM.
The pharmacovigilance survey opened following this mobilization, including also the hormonal device Jaydess, "has identified effects that should be mentioned in the leaflet for patients (asthenia, seborrhea)," the agency said this Friday, November 17 in a statement following a meeting of the Technical Committee of Pharmacovigilance (CTPV) of October 10.
Joint pain, erythema nodosum, psoriasis ... Upcoming investigations
The investigation "also revealed other signals requiring further investigations (via the continuation of the pharmacovigilance survey) such as arthralgia (pain in the joints), erythema nodosum (inflammation of the fatty tissue under -cutaneous), psoriasis and intracranial hypertension (HTIC). "
And to detail: "For the HTIC, an evaluation will be carried out later on the European level."
Overall monitoring of this type of IUD is also maintained.
No connection between the wearing of the IUD and anxiety "at this stage"
Another deleterious side effect often reported by the main concerned: the anxiety that would provoke the IUD.
In this regard, "very few cases had been reported before mid-May 2017. Anxiety cases were indeed massively reported after May 15, 2017: more than 900 cases were reported in total, including 870 after the 15 May 2017 ", stresses the ANSM which proceeded, in parallel with the analysis of the cases of pharmacovigilance, with a study" of the data of the Health Insurance to compare the frequencies of occurrence of certain undesirable effects between the carriers of IUD at levonorgestrel and copper IUDs. "
The result: "a low but increased risk of anxiolytic use in women with Mirena compared to copper IUD carriers."
Transmitted at the European level, these national results have not allowed, "at this stage", "to establish an association between the use of a levonorgestrel-containing IUD with isolated anxiety, panic attack, sleep or agitation ", we learn.
The European Medicines Agency (EMA) also states that "Mood disorders have been considered as related to depression or depressed mood, already mentioned in the documents intended for health professionals (RCP) and the women (notice). "
A further information document under development
An information document for women is currently being developed by ANSM, in "close collaboration with the association 'SVH ASSO Hormone Vigilance Hormones' ". The latter should be published and distributed before the end of the year.
In its press release, the agency recalls that Mirena is a drug and that its pose, carried out by a doctor or a midwife, must always be followed by the delivery of its notice.